The DEFINITIVE trial, funded by the European Commission, is a clinical study conducted across 44 hospitals in 7 European and associated countries. It aims to demonstrate that personalised treatment decisions in early-stage HER2-positive breast cancer, guided by the HER2DX® diagnostic assay, can improve patients’ quality of life while maintaining treatment efficacy and survival outcomes. It also seeks to prove that HER2DX® is safe and efficient, and will reduce direct and indirect costs for hospitals and public health systems.
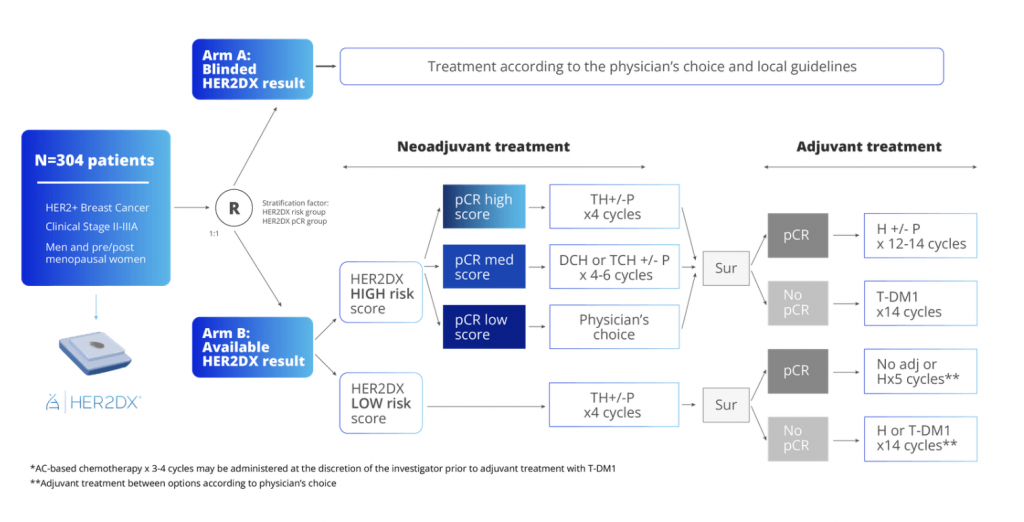
The DEFINITIVE assay is a 5-year international phase III clinical trial. As a two-arm randomised trial, it will include 304 patients with early-stage (stage II to IIIA) HER2-positive breast cancer who have not received previous treatment. These patients will be randomly divided into two groups to compare the outcomes:
The DEFINITIVE consortium is composed of 18 partners from 9 different countries, assuring European coverage and contribution through multidisciplinary expertise to the project’s main objectives.
The consortium has gathered world-known clinicians and key experts in breast cancer, as well as health policy and assessment experts, European patient organisations, and different associations such as Sharing Progress in Cancer Care (SPCC), to engage other regional, national, and European actors necessary for Europe wise uptake and implementation of HER2DX®.
Specific actions for policy making and joint meetings and activities with other EU Cancer initiatives and projects have been planned to inform policy makers about HER2DX® and the project results to overcome any potential political barrier.
SPCC is now developing the training of health professionals and the dissemination and communication activities of the project.
More to come.

Horizon Europe Grant Agreement n. 101136953 – Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.